What are indicators for?
Initially, the property of these compounds to change color in different environments was widely used to visually determine the acid-base properties of substances in solution, which helped to determine not only the nature of the environment, but also to draw a conclusion about the reaction products formed. Indicator solutions continue to be used in laboratory practice to determine the concentration of substances by titration and allow one to learn how to use available methods in the absence of modern pH meters.
There are several dozen substances of this kind, each of which is sensitive to a rather narrow area: usually it does not exceed 3 points on the information content scale. Thanks to such a variety of chromophores and their low activity among themselves, scientists were able to create universal indicators that are widely used in laboratory and industrial conditions.
ADSORPTION INDICATORS
Adsorption indicators are substances in the presence of which a change in the color of the precipitate formed during titration by precipitation occurs. Many acid-base indicators, some dyes and other chemical compounds are capable of changing the color of a precipitate at a certain pH value, which makes them suitable for use as adsorption indicators.
| Indicator | Ion to be detected | Ion precipitator | Color change |
| Alizarin red C | Yellow - pink-red | ||
| Bromophenol blue | Yellow - green | ||
| Lilac - yellow | |||
| Violet - blue-green | |||
| Diphenylcarbazide | , , | Colorless - violet | |
| Congo red | , , | Red - blue | |
| Blue - red | |||
| Fluorescein | , | Yellow-green - pink | |
| Eosin | , | Yellow-red - red-violet | |
| Erythrosine | Red-yellow - dark red |
Most used pH indicators
It is noteworthy that in addition to the identification property, these compounds have good coloring ability, which allows them to be used for dyeing fabrics in the textile industry. Of the large number of color indicators in chemistry, the most famous and used are methyl orange (methyl orange) and phenolphthalein. Most other chromophores are currently used in mixtures with each other, or for specific syntheses and reactions.
forms of application of indicators, pH measurement, sample analysis
Indicators are usually used by adding a few drops of an aqueous or alcoholic solution, or a little powder (for example, a mixture of murexide and sodium chloride) to a sample of the solution being tested.
During titration, an indicator is added to an aliquot of the test solution and the color changes at the equivalence point are observed.
Another method of application is to use strips of paper soaked in an indicator solution or a mixture of indicators and dried (Universal indicator).
Methyl orange
Many dyes are named due to their primary colors in a neutral environment, which is also inherent in this chromophore. Methyl orange is an azo dye that has a group – N = N – in its composition, which is responsible for the color of the indicator turning red in an acidic environment, and yellow in an alkaline environment. Azo compounds themselves are not strong bases, but the presence of electron-donating groups (‒ OH, – NH2, – NH (CH3), – N (CH3)2, etc.) increases the basicity of one of the nitrogen atoms, which becomes capable of attaching hydrogen protons in a donor manner. acceptor principle. Therefore, when the concentrations of H+ ions in the solution change, a change in the color of the acid-base indicator can be observed.
home > directory > chemical encyclopedia:Indicators
select the first letter in the article title:Indicators (late Lat. indicator - pointer), chemical substances that change color, luminescence or form a precipitate when the concentration of any component in the solution changes. Indicate a certain state of the system or the moment when this state is achieved.
There are reversible and irreversible indicators.
The change in color of the former when the state of the system changes can be repeated many times. Irreversible indicators undergo irreversible chemical transformations, for example, azo compounds are destroyed during oxidation by BrO3- ions. Indicators. which are introduced into the test solution are called internal, in contrast to external ones, the reaction with which is carried out outside the analyzed mixture. In the latter case, one or several drops of the analyzed solution are placed on a piece of paper soaked in an indicator, or they are mixed on a white porcelain plate with a drop of the indicator. Indicators are most often used to determine the end of a chemical reaction, mainly the end point of titration (e.t.t.). In accordance with titrimetric methods, acid-base, adsorption, redox and complexometric indicators are distinguished. Acid-base indicators are soluble organic compounds that change their color or luminescence depending on the concentration of H+ ions (pH of the medium). Application for determining the end of the reaction between acids and bases (including during acid-base titration) or other reactions if H+ ions are involved in them, as well as for the colorimetric determination of the pH of aqueous solutions. The most important acid-base indicators are given in table. 1. The reason for the change in color of indicators is that the addition or donation of protons by its molecules is associated with the replacement of some chromophore groups by others or with the appearance of new chromophore groups. If the indicator is a weak acid HIn, then in an aqueous solution there is an equilibrium: HIn + H2O D In- + H3O+. If the indicator is a weak base Shtb thenZh Sht + RBIGIYU2B.YGIYUSH V RShtBYGZYU + B.YGZYU + ONBYGZYU-B.YGZYU In general form we can write: Ina + H2O D Inb + H3O
+, where Ina and Inb are the acidic and basic forms of indicators, respectively , which are colored differently.
The equilibrium constant of this process K
ln
=
[Inb][H3O+]/[Ina] is called the indicator constant. The color of the solution depends on the ratio [Ina]/[Inb], which is determined by the pH of the solution.
The color of one form of the indicator is considered to be noticeable if its concentration is 10 times higher than the concentration of the other form, that is, if the ratio [Ina]/[Inb] = [H3O+]/Kln is 0.1 or 10. A change in the color of the indicator is noted in the area pH = pK
lп
b
1, which is called the indicator transition interval.
The change is most pronounced when [Ina] = [Inb] and Kln = [H3O]+, i.e. at pH = pKln. The pH value at which titration usually ends is called the titration index p T.
Indicators for titration are selected so that the color transition interval includes the pH value that the solution should have at the equivalence point. Often this pH value does not coincide with the pT of the indicator used, which leads to the so-called indicator error. If an excess of an untitrated weak base or acid remains in the c.t.t., the error is called. resp. basic or acidic. The sensitivity of indicators is the concentration (in mol/l) of the ion being determined (in this case, H+ or OH-) at the point of the most dramatic color transition. There are: acid-sensitive indicators with a transition interval in the region of alkaline pH values (eg, phenolphthalein, thymolphthalein); base-sensitive indicators with a transition interval in the acidic region (as in dimethyl yellow, methyl orange, etc.); neutral indicators, the transition interval of which is around pH 7 (neutral red, phenol red, etc.). Indicators come in one or two colored shapes; such indicators are called respectively. single-color and two-color. The most distinct color change would be observed in those indicators whose acid and base forms are colored in complementary colors. However, such indicators exist. Therefore, by adding dye, the colors of both forms are changed accordingly. Thus, for methyl red, the transition from red to yellow occurs in the range of 2 pH units, and if methylene blue is added to the solution, then the color transition from red-violet to green is observed sharply and clearly at pH 5.3. A similar effect can be achieved if you use a mixture of two indicators, the colors of which complement the other. friend. Such indicators are called mixed (Table 2).
Mixtures of indicators that continuously change their color over the entire pH range from 1 to 14 are called universal. They are used to roughly estimate the pH of solutions. The color change of the indicator is influenced by its concentration. For two-color indicators, the higher the concentration, the less dramatic the color change, since the absorption spectra of both forms overlap each other to a greater extent and the color change becomes more difficult to detect. Usually the same minimum (a few drops of solution) amount of indicator is used.
The transition interval of many indicators depends on temperature.
Thus, methyl orange changes its color at room temperature in the pH range of 3.4-4.4, and at 100 °C in the pH range of 2.5-3.3. This is due to a change in the ionic product of water. The colloidal particles present in the solution adsorb indicators, which leads to a complete change in its color. To eliminate errors, in the presence of positively charged colloidal particles, base indicators should be used, and in the presence of negatively charged ones, acid indicators should be used. When titrating under normal conditions, it is necessary to take into account the influence of dissolved CO2, especially when using indicators with pK
ln
>
4 (for example, methyl orange, methyl red, phenolphthalein.. Sometimes CO2 is first removed by boiling or the solution is titrated in the absence of contact with the atmosphere. The influence of extraneous neutrals electrolytes (salt effect) manifests itself in a shift in the equilibrium of indicators. In the case of acid indicators, the transition interval shifts to a more acidic region, and in the case of base indicators, to a more alkaline region. Depending on the nature of the solvent, the colors of the indicators, their
pK
ln and sensitivity change. Thus, methyl red in water gives a color transition at higher values of H+ ion activity than bromophenol blue, and in an ethylene glycol solution, vice versa. In water-methanol and water-ethanol solutions, the change compared to an aqueous medium is insignificant. In an alcohol medium, the indicators are acids are more sensitive to H+ ions than base indicators. Although when titrating in non-aqueous media, the temperature is usually set potentiometrically using a glass indicator electrode. Acid-base indicators are also used (Table 3). Most often, methyl red in dioxane or crystal violet in anhydrous CH3COOH are used for titration of weak bases; when titrating weak acids - thymol blue in DMF. The behavior of indicators in non-aqueous and aquatic environments is similar. For example, for a weak acid HIn in a solvent SH, the equilibrium can be written: HIn + SH D In- + SH2+. The mechanism of action of indicators is the same as in water, only in non-aqueous media the corresponding acidity scales (pHr) are used. Luminescent indicators are also used as acid-base indicators. changing color and fluorescence intensity depending on pH and allowing the titration of highly colored and turbid solutions.
For the titration of weak acids, so-called turbidity indicators of the substance are used, forming reversible colloidal systems that coagulate in a very narrow pH range (for example, isonitroacetyl- n
-aminobenzene gives turbidity at pH 10.7-11.0).
Metal complexes with metallochromic indicators (see below) can be used as acid-base indicators; These complexes, when destroyed, change the color of the solution in a narrow pH range. To determine organic acids and bases in water in the presence of an immiscible solvent, so-called amphi indicators are used, which are salts of acid indicators (for example, tropeolin 00) with decomp.
org. bases (eg alkaloids). These indicators are highly soluble in organic solvents and poorly soluble in water; are highly sensitive. Adsorption indicators are substances that can be adsorbed on the surface of a sediment and thereby change the color or intensity of luminescence. These indicators, as a rule, are reversible and are used in precipitation titrations. First of all, ions identical to those that are part of the sediment itself are adsorbed by the precipitate, after which the indicators are adsorbed. A large group of indicators are dyes (Table 4), which are adsorbed by the surface of the sediment to form salts with the ions contained in the sediment. For example, a solution of eosin is pink in color, which does not change when AgNO3 is added. But when titrated with a KBr solution, the precipitate that appears adsorbs Ag+ ions, which attach eosin anions. The precipitate becomes red-violet. At the c.t.t., when all Ag+ ions have been titrated, the color of the precipitate disappears and the solution becomes pink again. Inorganic adsorption indicators form a colored precipitate or complex with titrant ions (such as, for example, CrO4- and SCN- ions used as indicators in argentometry). Some acid-base, redox and complexometric indicators are also used as adsorption indicators, the properties of which (acid dissociation constants, redox potentials and stability constants of complexes with metal cations) in the adsorbed state depend on the nature and concentration of ions on the surface of the sediment.
Redox indicators are substances that can change color depending on the redox potential of the solution.
They are used to establish the temperature of redox titration and for the colorimetric determination of redox potential (mainly in biology). Such indicators are, as a rule, substances that themselves undergo oxidation or reduction, and the oxidized (InOx) and reduced (InRed) forms have different colors. For reversible redox indicators, we can write: InOх + ne
D InRed, where
n is
the number of electrons.
At potential E,
the ratio of concentrations of both forms of indicators is determined by the Nernst equation:
Where Eln is the real redox potential of the indicator, depending on the composition of the solution. The color transition interval is practically observed when the ratio [In0x]/[InRed] changes from 0.1 to 10, which at 25 °C corresponds to DE (in V) = Eln b (0.059/ n
). The potential corresponding to the sharpest color change is Eln. When choosing indicators, the main factors taken into account are the Eln values, the molar extinction coefficient of both forms of indicators, and the potential of the solution at the point of equivalence of the indicators. When titrating with strong oxidizing agents (K2Cr2O7, KMnO4, etc.), indicators with relatively high Eln are used, for example, diphenylamine and its derivatives; when titrating with strong reducing agents [salts Ti(III), V(II), etc.], indicators with relatively low Eln are used, for example, safranin, methylene blue (Table 5).
Some substances change their color irreversibly, for example, during oxidation they are destroyed to form colorless products, such as indigo under the influence of hypochlorites or naphthol blue-black under the influence of BrO3 ions.
Complexometric indicators are substances that form colored complexes with metal ions (M), differing in color from the indicators themselves.
They are used to establish c.t.t. in complexometry. The stability of metal complexes with indicators (In) is less than that of the corresponding complexonates, therefore, in complex conditions, complexons displace indicators from complexes with metals. At the moment of color change at the equivalence point [In] = [MIn] and, therefore, рМ = - log KMln, where рМ = - log[M] is called. indicator
transition point , KMln is the stability constant of the metal complex with the indicator.
Error in titration occurs because some metal ion may attach to the indicator rather than to the titrant. The most commonly used are the so-called metallochromic indicators (Table 6) - org. substances that form, with metal cations, intensely colored (el 104-105) intracomplex compounds soluble in water. These compounds must be stable enough to form at very low concentrations of metal ions. The indicator and its complex must be in true solution. To increase the solubility of the reagent and complex, a water-miscible solvent is usually introduced. The metal complex with the indicator must be labile and quickly destroyed by the action of the complexone. There are selective and universal metallochromic indicators that interact respectively. with few or many cations. The former include, for example, tyrone,
gallione, the latter
- arsenazo
I,
pyridylazonaphthol
(PAN),
pyrocatechol violet, xylenol orange, methylthymol blue, chromasurol
, etc.
indicator is used as complexometric indicators
, for example PAN.
When the detectable ion M2+ is introduced, the reaction occurs: M2+ + [CuY]2- + PAN D [MY]2- + [CuPAN]+. When titrated with any complexone in room temperature, the color of the solution changes from violet to yellow-orange, i.e. very contrasting. In the complexometric determination of Cu, its complex with PAN adsorbed on the surface of deposited AgI is used as an indicator. In this case, it is possible to determine Ag and Cu in their joint presence: first Ag ions are titrated with a KI solution in an acidic medium, and then Cu ions are titrated with a complexone. So-called uncolored complexometric indicators are also used, which selectively interact with ions of the metal being determined to form weakly colored (el 103) complexes, for example, sulfosalicylic acid
when titrating Fe (III).
Fluorescent complexometric indicators (or metal fluorescent indicators) interact with metal cations to form intensely fluorescent chelates. Indicators were first reported by the English physicist and chemist R. Boyle in 1664. Lit.:
Indicators, trans.
from English, vol. 1-2, M., 1976; Denesh I., Titration in non-aqueous media, trans. from English, M., 1971; Korenman I.M., New titrimetric methods, M., 1983. L.N. Simonova.
select the first letter in the article title:
Learn more about making methyl orange
Methyl orange is obtained by reaction with diazotization of sulfanilic acid C6H4(SO3H)NH2 followed by combination with dimethylaniline C6H5N(CH3)2. Sulfanilic acid is dissolved in a sodium alkali solution by adding sodium nitrite NaNO2, and then cooled with ice to carry out the synthesis at temperatures as close as possible to 0°C and hydrochloric acid HCl is added. Next, prepare a separate solution of dimethylaniline in HCl, which is poured cooled into the first solution to obtain a dye. It is further alkalized, and dark orange crystals precipitate from the solution, which after several hours are filtered and dried in a water bath.
How to manage color
Color management apps, like other third-party Android apps, are recommended to be downloaded from the Google Play Store. We offer two options.
Light Manager
This is a completely free application that can only bother the user with advertising banners. To get rid of them, you need to update the utility to the paid version.
First of all, Light Manager needs to provide access to all notifications. The application itself will prompt you to do this. Next you need to do this step by step:
- go to “Settings”;
- select the “Ringtones and notifications” tab;
- select the “Access to notifications” tab;
- check the box next to Light Manager.
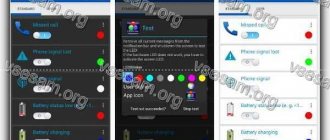
Settings are organized into categories that allow you to customize colors and flashing patterns. If you don't see a color next to an app, it's not configured and is the default. Activation, color change and deactivation of notifications occur as a result of pressing.
Light Flow Pro
This application works similar to the previous one. It provides the option to make notifications cyclic or show priority. It supports up to 6 hundred instruments, but the lightweight free version (Light Flow Lite) can change the color of the LEDs only for the most important functions. The utility requests permission to access applications, which does not suit all users.
Unlike the “Manager” there are means of manipulating sound and vibration. Not compatible with all devices. The developer gives the example of NTS smartphones, which support only three colors and therefore their functionality is limited. Gadgets from other brands can also surprise you. The LEDs may not flash or stay on all the time. If you encounter problems with the interaction between the application and the device, it is recommended to find “Direct mode” in the settings. When activated, it controls the LED flashlight.
After granting access permission, you need to open “Notification” on the main screen of the application. It will contain a list of events, the display of which can be configured at your discretion. After selecting the type of setting, notifications will be divided into tabs. You can set your own colors.
Light notifications are not the most important, but still useful feature for Android devices. They effectively help to recognize the type of notification. Even if the smartphone is in standby mode and with the sound turned off, the user will be able to understand its signals and respond.
Phenolphthalein
This chromophore got its name from adding the names of two reagents that are involved in its synthesis. The color of the indicator is notable for its color change in an alkaline environment with the acquisition of a crimson (red-violet, crimson-red) hue, which becomes discolored when the solution is strongly alkalized. Phenolphthalein can take several forms depending on the pH of the environment, and in strongly acidic environments it has an orange color.
This chromophore is obtained by condensation of phenol and phthalic anhydride in the presence of zinc chloride ZnCl2 or concentrated sulfuric acid H2SO4. In the solid state, phenolphthalein molecules are colorless crystals.
Previously, phenolphthalein was actively used in the creation of laxatives, but gradually its use was significantly reduced due to the established cumulative properties.
REDOX INDICATORS
Redox indicators are chemical compounds that change the color of a solution depending on the value of the redox potential. They are used in titrimetric methods of analysis, as well as in biological studies for the colorimetric determination of redox potential.
| Indicator | Normal redox potential (at pH=7), V | Coloring the solution | |
| oxidative form | restored form | ||
| Neutral red | —0,330 | Red-violet | Colorless |
| Safranin T | —0,289 | Brown | Colorless |
| Potassium indigomonosulfonate | —0,160 | Blue | Colorless |
| Potassium indigodisulfonate | —0,125 | Blue | Colorless |
| Potassium indigotrisulfonate | —0,081 | Blue | Colorless |
| Potassium indigo tetrasulfonate | —0,046 | Blue | Colorless |
| Toluidine blue | +0,007 | Blue | Colorless |
| Tnonin | +0,06 | Purple | Colorless |
| Sodium o-Cresolindophenolate | +0,195 | Reddish blue | Colorless |
| Sodium 2,6-Dnchlorophenolindophenolate | +0,217 | Reddish blue | Colorless |
| Sodium m-bromophenolindophenolate | +0,248 | Reddish blue | Colorless |
| Diphenylbenzidine | +0.76 (acidic solution) | Purple | Colorless |
Litmus
This indicator was one of the first reagents used on solid media. Litmus is a complex mixture of natural compounds that is obtained from certain types of lichens. It is used not only as a dye, but also as a means for determining the pH of the environment. This is one of the first indicators that began to be used by humans in chemical practice: it is used in the form of aqueous solutions or strips of filter paper soaked in it. Solid litmus is a dark powder with a faint ammonia odor. When dissolved in clean water, the color of the indicator takes on a violet color, and when acidified it turns red. In an alkaline environment, litmus turns blue, which allows it to be used as a universal indicator for the general determination of environmental indicators.
It is not possible to accurately establish the mechanism and nature of the reaction that occurs when pH changes in the structures of litmus components, since it can contain up to 15 different compounds, some of which may be inseparable active substances, which complicates their individual studies of chemical and physical properties.
Setting the indicator light color
Despite the fact that the color of the indicator is defined by default, you can customize it, that is, change it as you wish
.
Unfortunately, this function is not present in all phones in the line. More expensive, modern and functional models (for example, Xiaomi Redmi Note 4X
or
Xiaomi Redmi Note 5
) have the ability to make the indicator light acquire different colors in accordance with the user’s preferences. The color palette depends on the gadget model. How to change the color of the application indicator?
- Open “Settings” in any convenient way.
- Go to “All applications” in the “Applications” section.
We select any application for which we want to install a light indication and click on it.
Scroll through and find “Notifications”. Click.
Find “Indicator light”. Turn it on. If possible (in our case not), select the color for displaying notifications from this application.
To change the color of the indication of calls, messages and notifications from the device, you need to go to the “Indicator Light” section (again, it is not available on all models!) and under the functions of turning on the LED, select the desired color for each of the categories.
LED Control keywords
in the name and expand the capabilities of the gadget.
Universal indicator paper
With the development of science and the advent of indicator papers, the establishment of environmental indicators was greatly simplified, since now there was no need to have ready-made liquid reagents for any field research, which is still successfully used by scientists and criminologists. Thus, solutions were replaced by universal indicator papers, which, due to their wide spectrum of action, almost completely eliminated the need to use any other acid-base indicators.
The composition of impregnated strips may differ from one manufacturer to another, so an approximate list of included substances may be as follows:
- phenolphthalein (0-3.0 and 8.2–11);
- (di)methyl yellow (2.9–4.0);
- methyl orange (3.1–4.4);
- methyl red (4.2–6.2);
- bromothymol blue (6.0–7.8);
- α-naphtholphthalein (7.3–8.7);
- thymol blue (8.0–9.6);
- cresolphthalein (8.2–9.8).
The packaging must contain color scale standards that allow you to determine the pH of the environment from 0 to 12 (about 14) with an accuracy of one whole integer.
Among other things, these compounds can be used together in aqueous and aqueous-alcoholic solutions, which makes the use of such mixtures very convenient. However, some of these substances may be poorly soluble in water, so it is necessary to select a universal organic solvent.
Due to their properties, acid-base indicators have found their use in many fields of science, and their diversity has made it possible to create universal mixtures that are sensitive to a wide range of pH values.
FLUORESCENT INDICATORS
Some chemical compounds, when exposed to ultraviolet rays, have the ability, at a certain pH value, to cause fluorescence of a solution or change its color or shade.
This property is used for acid-base titration of oils, cloudy and highly colored solutions, since conventional indicators are unsuitable for these purposes.
Work with fluorescent indicators is carried out by illuminating the test solution with ultraviolet light.
| Indicator | pH range of fluorescence change (in ultraviolet light) | Fluorescence color change |
| 4-Ethoxyacridone | 1,4-3,2 | Green - blue |
| 2-Naphthylamine | 2,8—4,4 | Increase in violet fluorescence |
| Dimetnlnaphteyrodine | 3,2—3,8 | Lilac - orange |
| 1-Naftilamnn | 3,4-4,8 | Increase in blue fluorescence |
| Acridine | 4,8—6,6 | Green - purple |
| 3,6-Dioxyphthalimide | 6,0—8,0 | Yellow-green - yellow |
| 2,3-Dicyanhydroquinone | 6,8—8,8 | Blue; green |
| Euchrysin | 8,4—10,4 | Orange - green |
| 1,5-Naphthylaminesulfonamide | 9,5—13,0 | Yellow green |
| CC acid (1,8-aminonaphthol 2,4-disulfonic acid) | 10,0-12,0 | Purple - green |
How to turn on the indicator light on Huawei and Honor
How to turn on the indicator on Honor and Huawei? To activate the event indicator, you must do the following:
- Go to settings.
- Go to the “Applications and Alerts” tab.
- Go to the Notifications and Status Bar section.
- Activate the "Blinking" option.
After the presented procedure, if events are missed, the LED will light up in the appropriate shade. For example, when charging, it will notify you about the battery status; if a call or message is not answered, it will start flashing green.
Decoding signals
Each manufacturer provides a specific decoding of the indicator light signals for Honor 10 Lite or other models. You need to know these features before changing the shade of the light bulb.
Basic Rules:
- Red. Indicates that the battery charge has decreased below the 10 percent mark. Honor smartphone requires fast charging. Second option - the device is charging, but the charge level is below 90% (applies only to two-color options)
- Orange flashing. The device is charging, and the charge level is from 10 to 90%. A message has arrived about a new notification (applies to two-color models).
- Green. The color of the indicator is characteristic of the charging mode. He confirms that the phone received more than 90%.
- Orange. Lights up while charging and indicates battery capacity is between 10 and 90 percent. No notifications. Relevant for 3 color phones.
- Flashing green. Indicates that you have received a message or missed a call. The charge level is more than 4%. If the green light is on while charging, this indicates a message has been received and the charge is greater than 90%.
Knowing the above signals, you can interpret the color of the indicator on Honor and understand what this or that shade means.
How to setup
To configure the function in question, you will need to contact a specific application. Let's say to change the settings for Whatsapp:
- Log in to messenger.
- Click on the three dots at the top to bring up the menu and select the Settings tab.
- Click on “Alerts” – “Light”.
- Choose the option you like, for example, blue.
How to reset an Honor and Huawei phone to factory settings: do a “hard reset”
If the smartphone does not support the selected shade, the light will blink white. This way the user will determine which program sent the message without removing the screen lock.
CHEMILUMINESCENT INDICATORS
This group of indicators includes substances that can emit visible light at certain pH values. Chemiluminescent indicators are convenient to use when working with dark liquids, since in this case a glow appears at the end point of the titration.
| Indicator | pH of the beginning of the glow |
| Dimethylbisacridene | About 9.0 |
| Lofin | 8,9—9,4 |
| Luminol | 8,0—8,5 |
| Lucigenin | 9,0-10,0 |
- How to make a cooling mixture >>